Nitrogen Element With Reaction, Properties, Uses, & Price Periodic Table

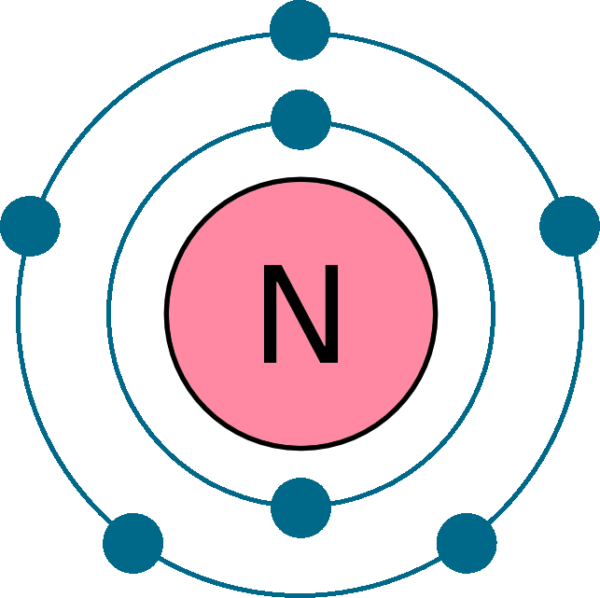
Orbital Diagram For Nitrogen (N) Nitrogen Electron Configuration
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.
Valence Electrons & Electron Configurations YouTube
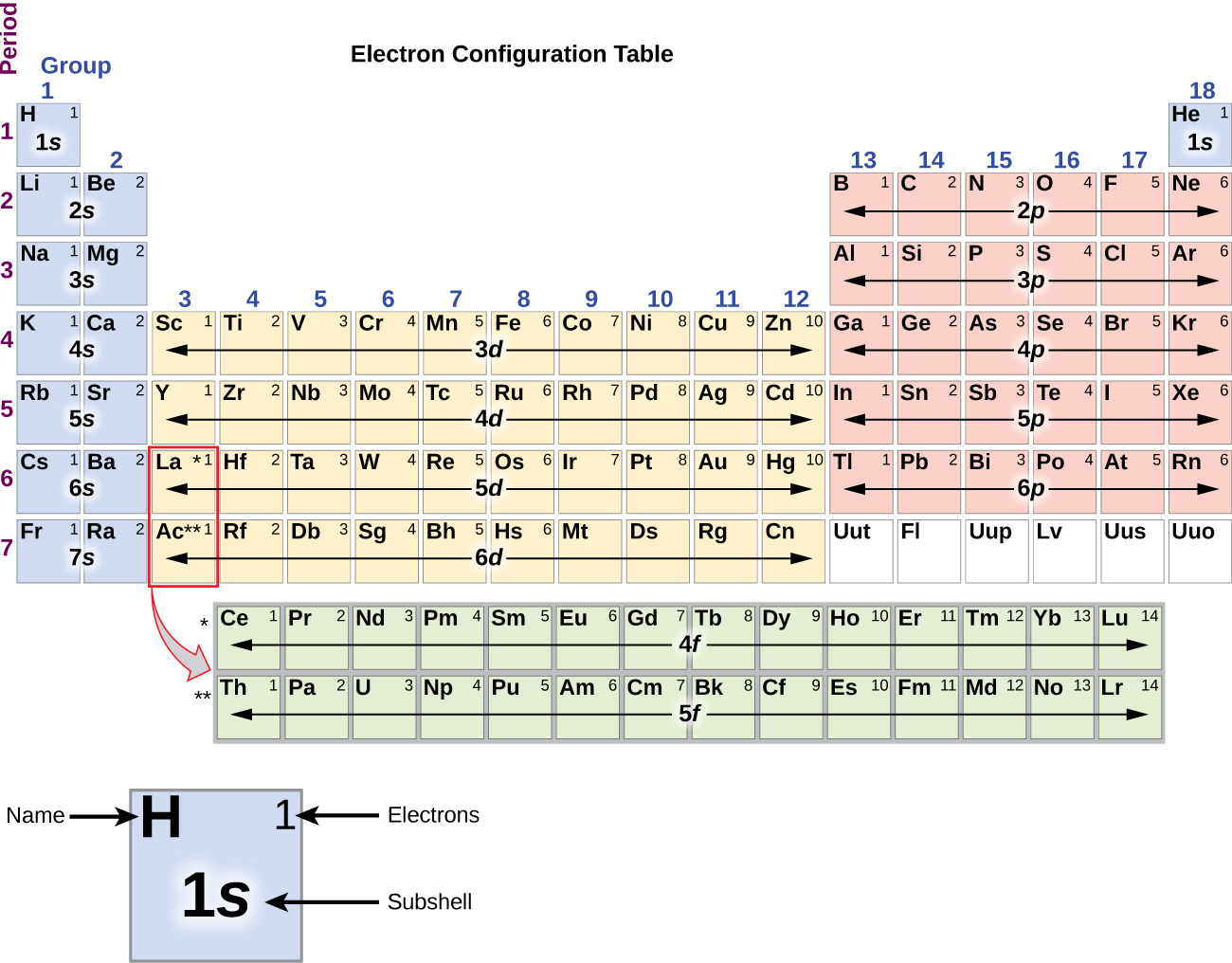
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 3.1.2 3.1. 2 ): The number of the principal quantum shell, n, The letter that designates the orbital type (the subshell, l ), and
Electron Configurations YouTube
Electron configurations are a simple way of writing down the locations of all of the electrons in an atom. As we know, the positively-charged protons in the nucleus of an atom tend to attract negatively-charged electrons.

Predict the Ground State Electron Configuration Co3+ Denker Ensterly
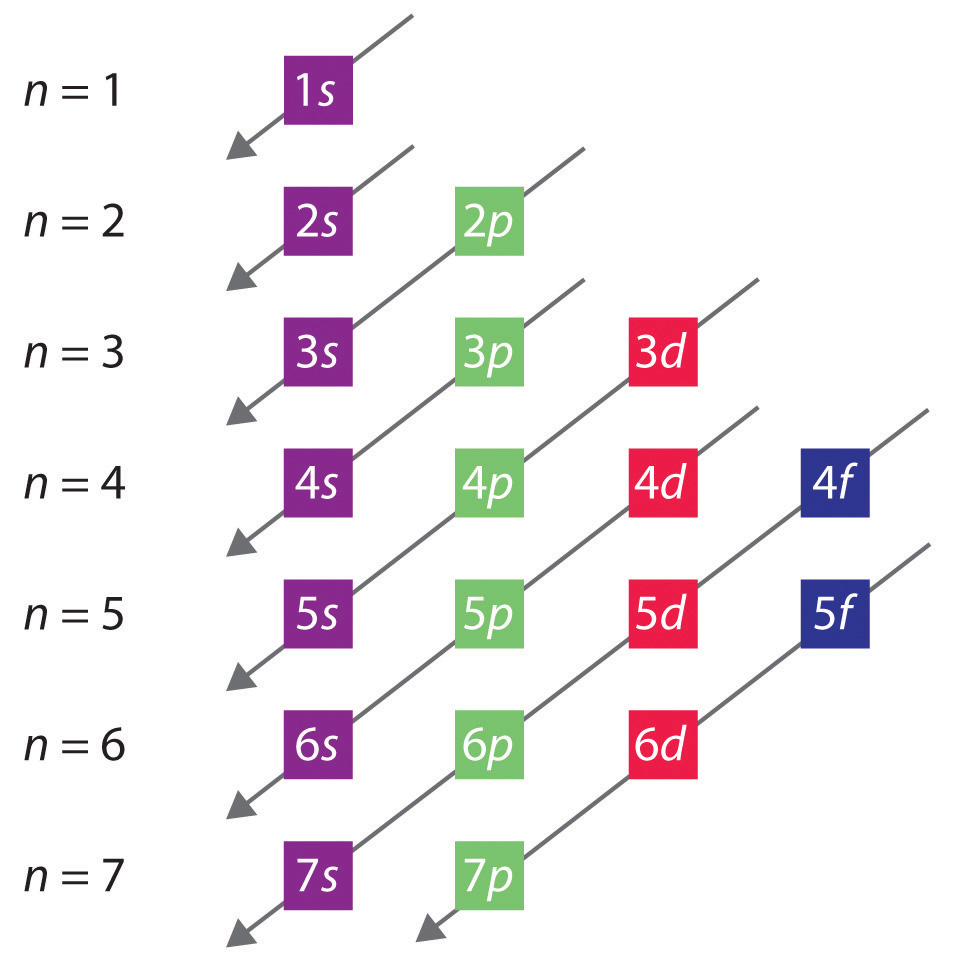
Answer: The electron configurations of the elements are presented in Figure 2.2.3, which lists the orbitals in the order in which they are filled. In several cases, the ground state electron configurations are different from those predicted by Figure 2.2.1. Some of these anomalies occur as the 3 d orbitals are filled.
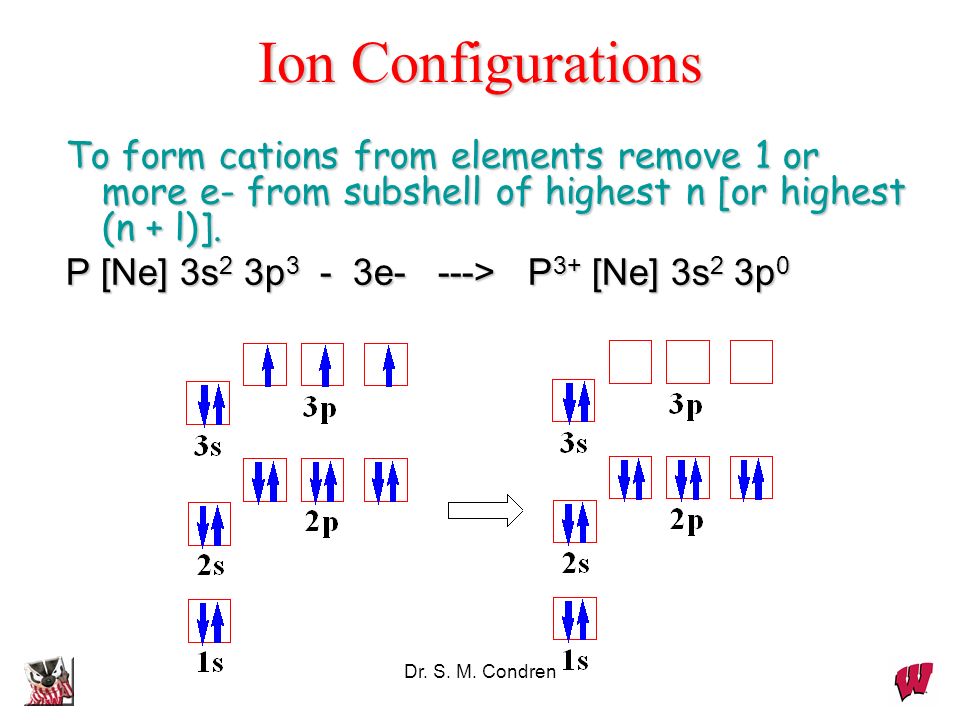
P [Ne] 3s2 3p3 3e —> P3+ [Ne] 3s2 3p0. Dr. S. M. Condren. Dynamic
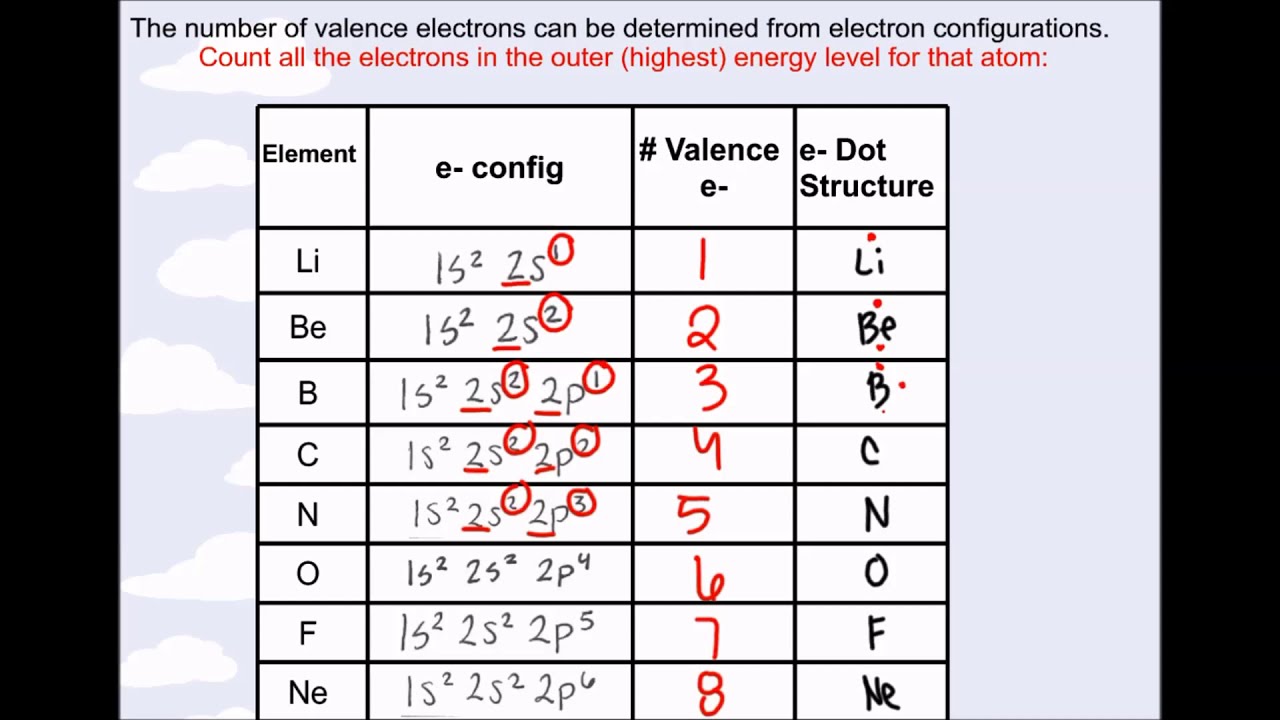
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 2p^4# F #1s^2 2s^2 2p^5#
/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)
Electron Configuration Chart
Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons.
Question 9267e Socratic
Principal Quantum Number (n) The principal quantum number n indicates the shell or energy level in which the electron is found. The value of n can be set between 1 to n, where n is the value of the outermost shell containing an electron. This quantum number can only be positive, non-zero, and integer values. That is, n=1,2,3,4,.. For example, an Iodine atom has its outmost electrons in the 5p.
6.4 Electronic Structure of Atoms (Electron Configurations
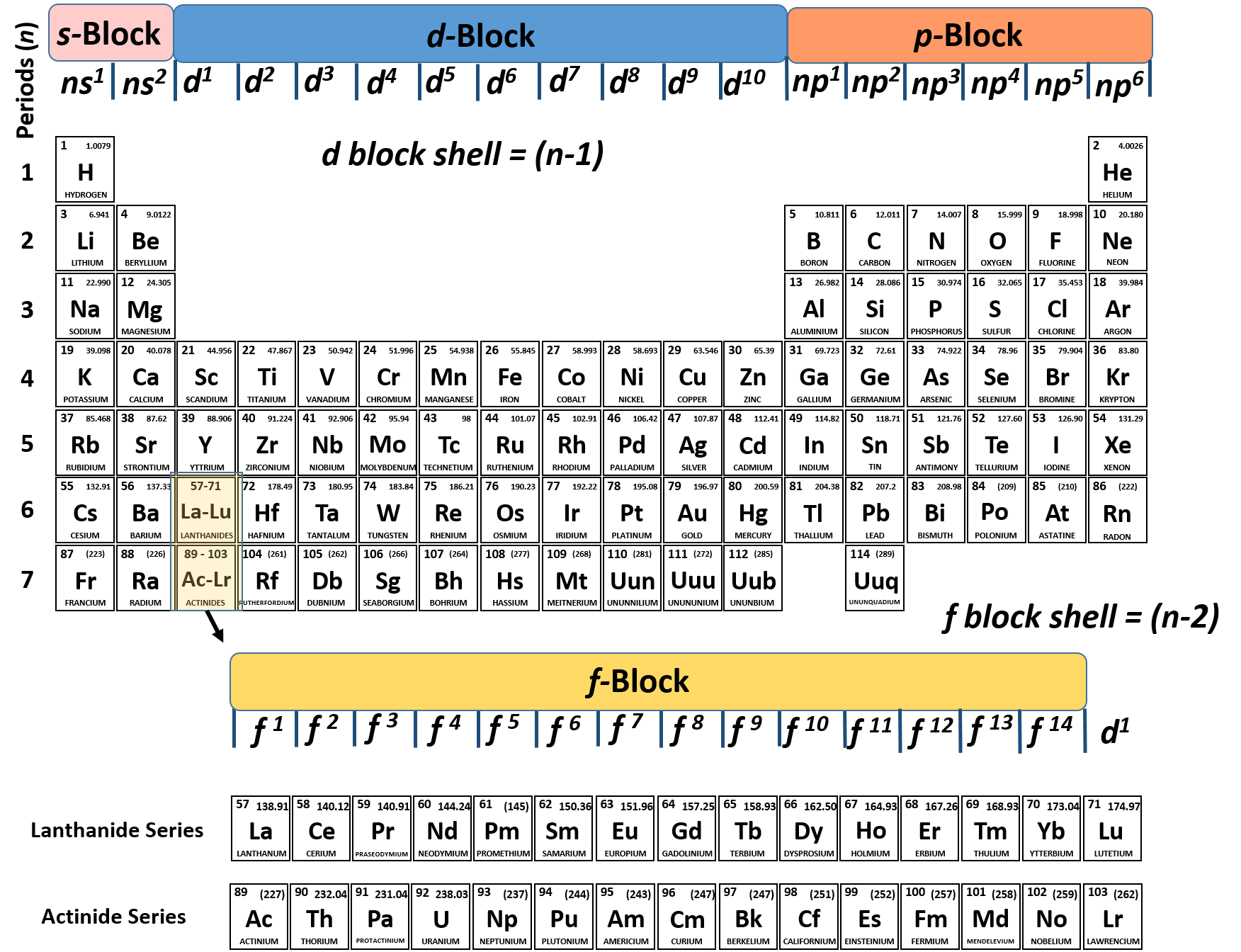
The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a noble gas, but the loss of two.
Printable Periodic Table Of Elements With Electron Configuration Pdf
AboutTranscript. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Created by Sal Khan.
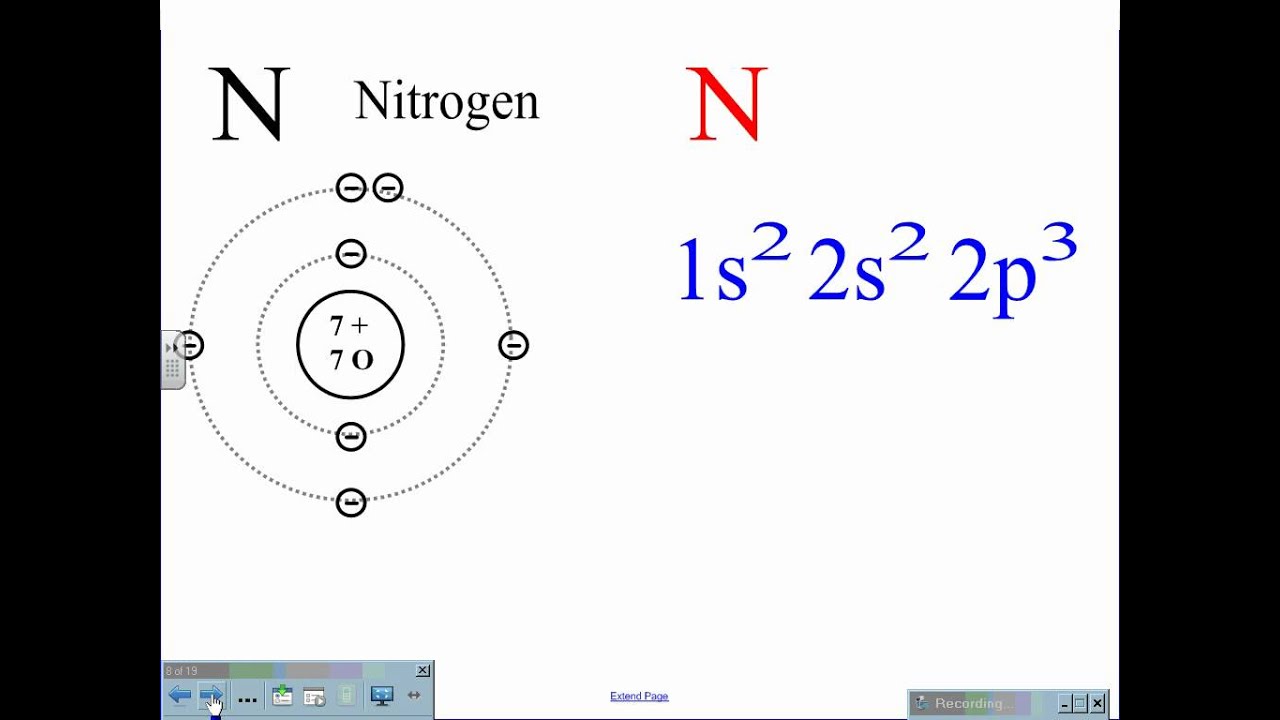
Use The Orbital Diagram For Nitrogen To Write Quantum Numbers For The
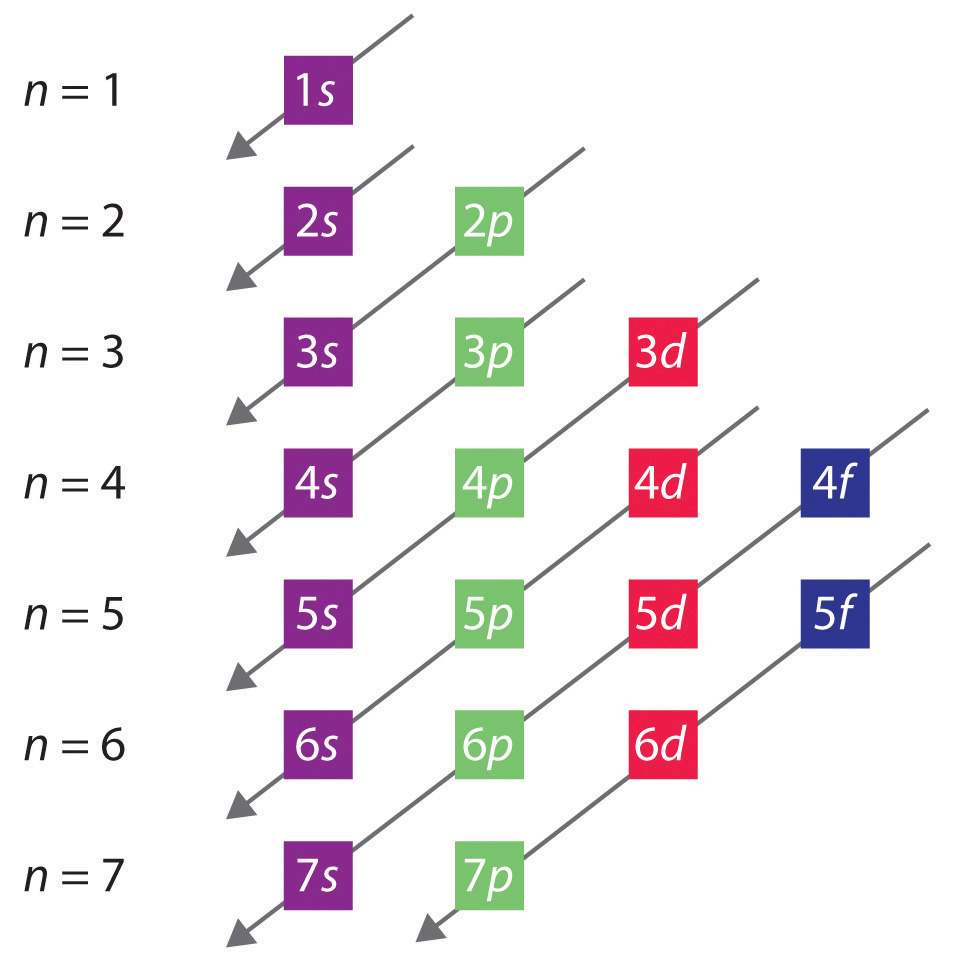
When writing the electron configuration for an atom, orbitals are filled in order of increasing atomic number. However, there are some exceptions to this rule. Example 3: 3 rd row elements. Following the pattern across a period from B (Z=5) to Ne (Z=10), the number of electrons increases and the subshells are filled.

Electronic Configurations Intro Chemistry LibreTexts
Electron configuration provides insight into the chemical behaviors of elements and is an important concept for students to master in introductory chemistry. To better strengthen undergraduate students' mastery of electron configurations of atoms and ions, we designed a novel, interactive chemistry game called ChemisTree that uses active-learning techniques (e.g., physically building.
Nitrogen Element With Reaction, Properties, Uses, & Price Periodic Table
The numbers of electrons that can occupy each shell and each subshell arise from the equations of quantum mechanics, [a] in particular the Pauli exclusion principle, which states that no two electrons in the same atom can have the same values of the four [2]

Electron Configuration For Manganese Atomic Number 25 How Do You Draw
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,
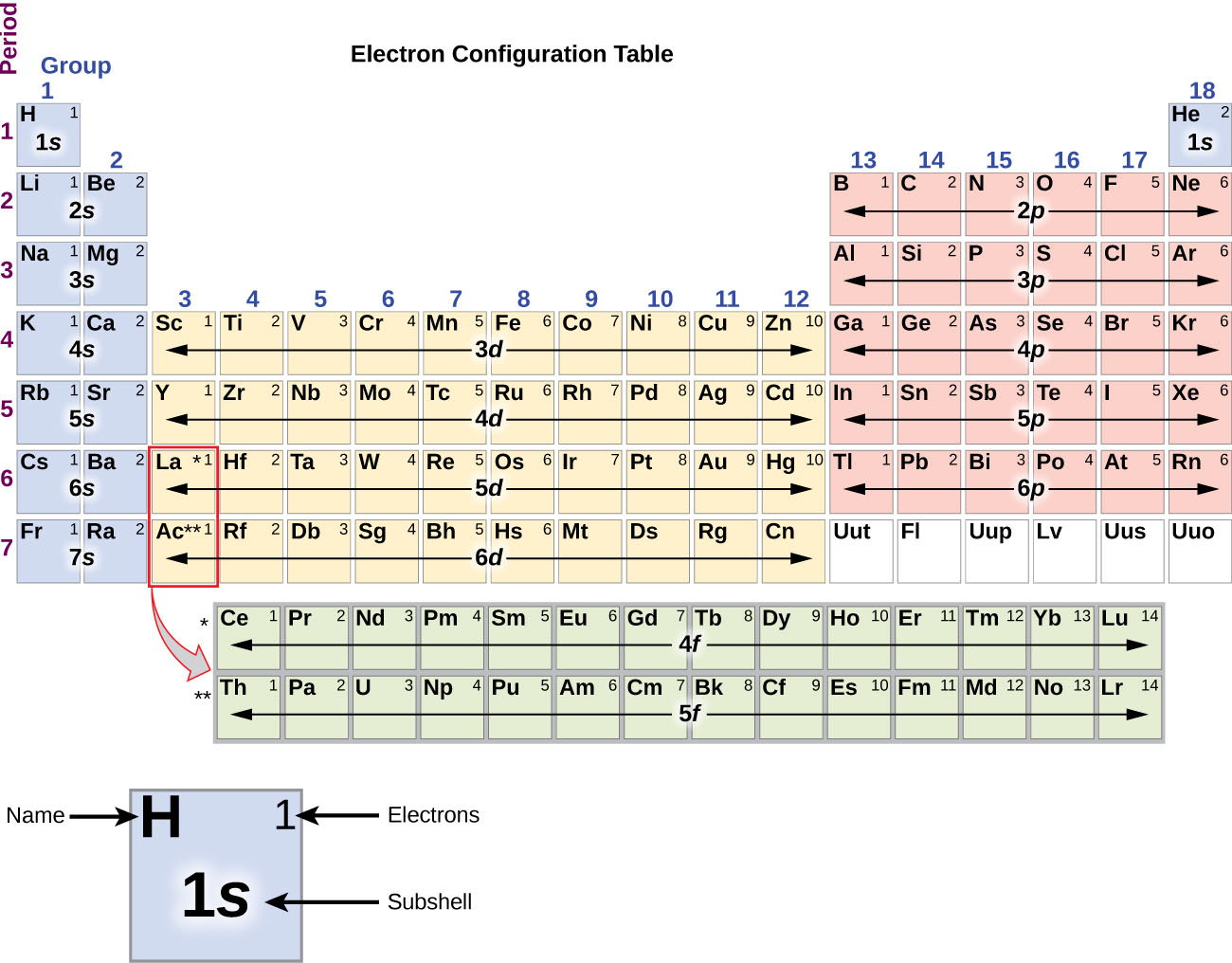
M7Q7 Electron Configurations, Orbital Box Notation Chem 103/104
The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and 14. Thus in the building-up process for the lanthanoids.
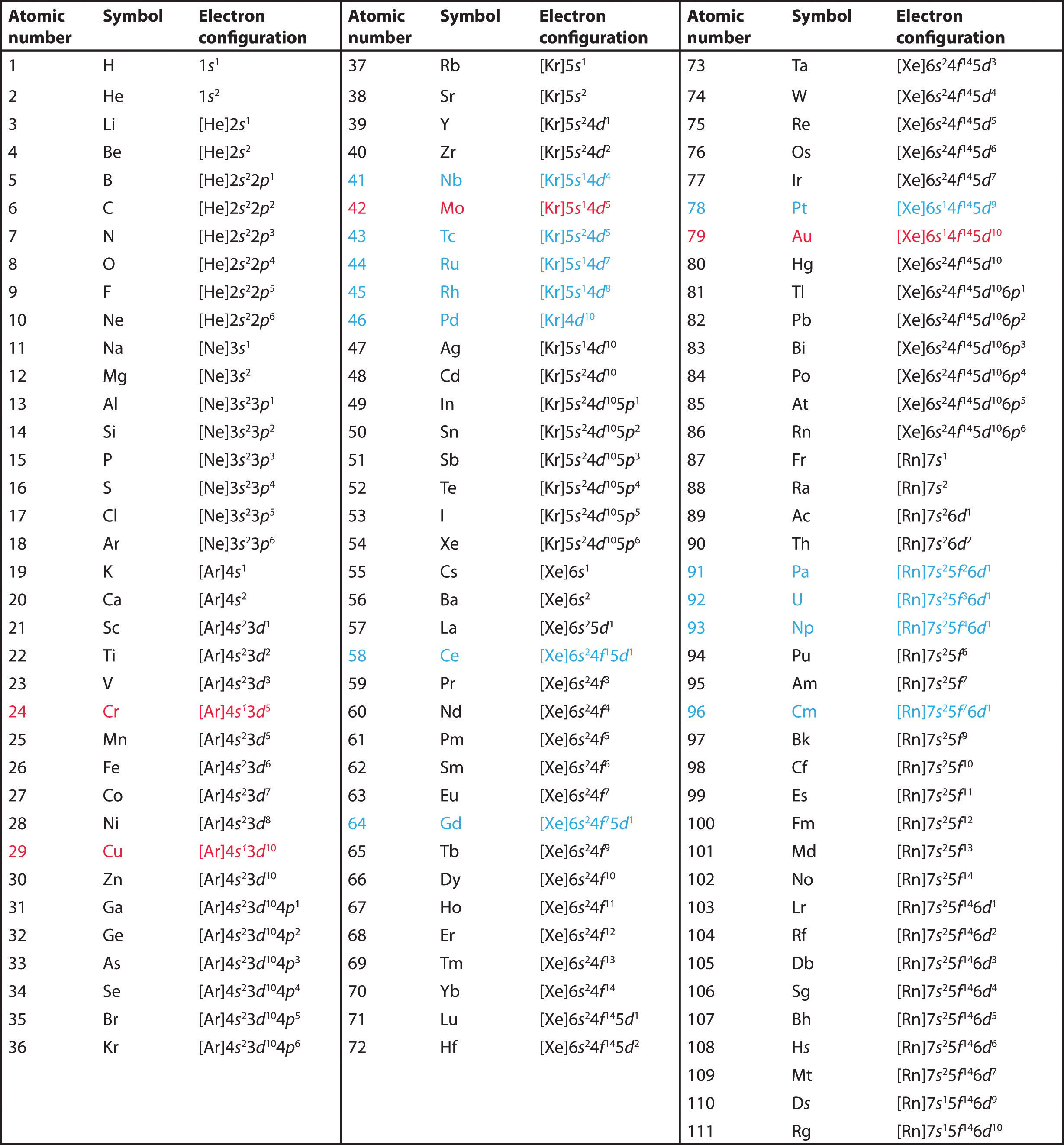
7.8B Electron Configurations and the Periodic Table Chemistry LibreTexts
Electron Configurations are useful for: Determining the valency of an element. Predicting the properties of a group of elements (elements with similar electron configurations tend to exhibit similar properties). Interpreting atomic spectra.
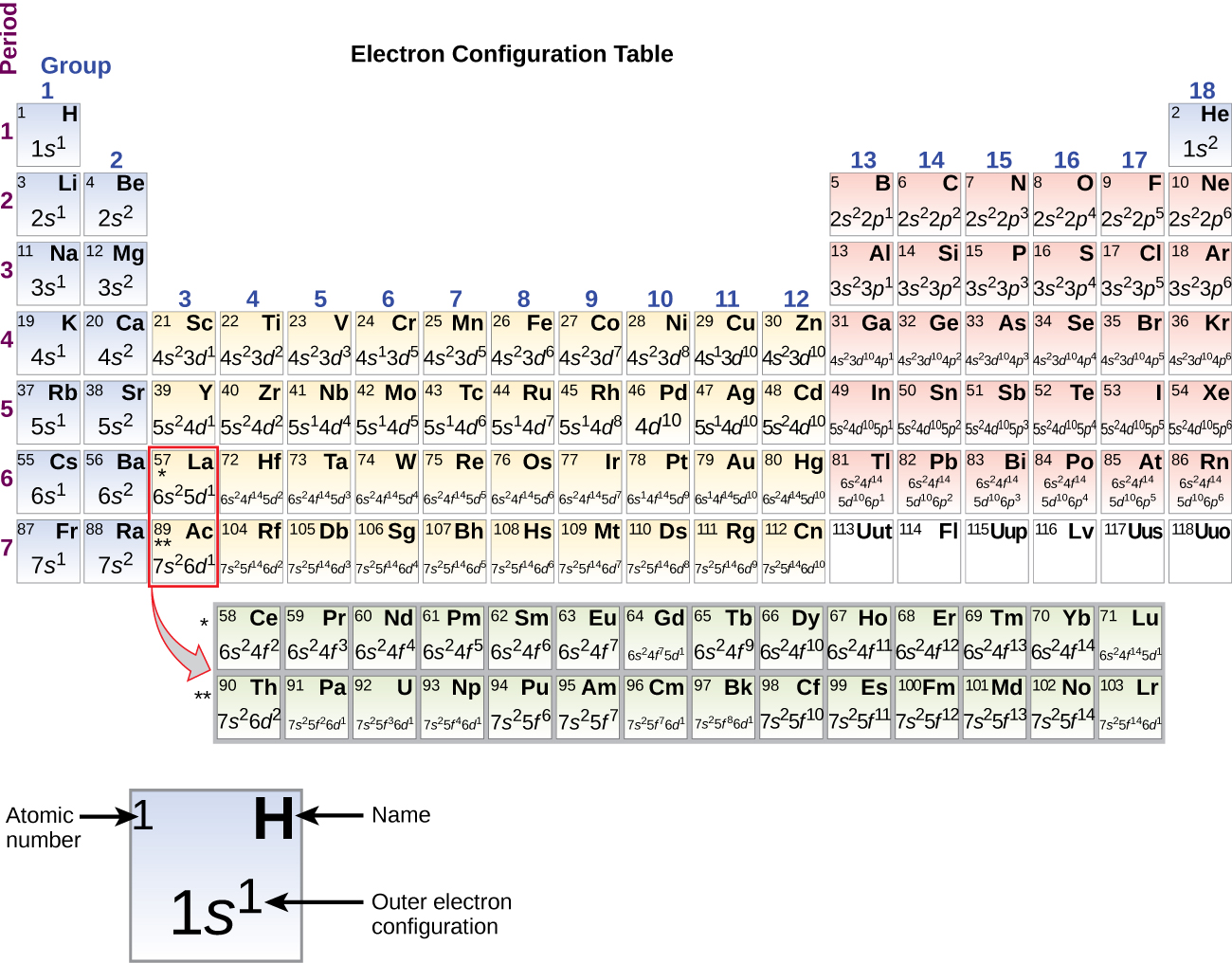
Electron Configurations, Orbital Box Notation (M7Q7) UWMadison
The electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements contains all the elements in increasing order of atomic number. To save room, the configurations are in noble gas shorthand.