Green Calcite calcium carbonate CaCO3 Stock Photo Alamy

Full carbonate chemistry at the site of calcification in a tropical coral
For example, this chart gives the bulk density ratio of baking soda:calcium carbonate as about 1.13:1 (actually, 801:700). Then, you could say that baking soda is about 52% CO2, while calcium carbonate is about 44% CO2. Combining those numbers, we should use about 35% more calcium carbonate to get the same leavening effect.

Calcium Carbonate Production
Food additives with strong water-absorbing capacity were patent protected, including calcium carbonate, calcium sulphate, diatomaceous earth and fruit peels. Table 1. Neutral fillers for chemical leavening, with patent priority date * Compound. Leavening acids like tartrates accelerated flour rancidity (Watts et al., 1949).

E170 Calcium Carbonate This Nutrition
Calcium carbonate may cause serious side effects. Call your doctor at once if you have: little or no urinating; swelling, rapid weight gain; or. high levels of calcium in your blood-- nausea, vomiting, constipation, increased thirst or urination, muscle weakness, bone pain, confusion, lack of energy, or feeling tired.

NEW Bubble & Bee Alkalizing Toothpaste LEMON Bubble and Bee Organic
Calcium carbonate, with its slower leavening action, creates a more tender and moist texture, which is ideal for cakes and quick breads. Beyond Baking: Other Uses. While both calcium carbonate and baking soda excel in the world of baking, they also have their own unique uses outside of the oven. Let's explore the versatile nature of these.

Green Calcite calcium carbonate CaCO3 Stock Photo Alamy
The prevalent baking acids in modern chemical leavening systems are sodium or calcium salts of ortho, pyro, and complex phosphoric acids. Baking soda determines the amount of carbon dioxide evolved, whereas the type of acid controls the speed of liberation. By far, the greatest use of leavening in the home is in preleavened mixes..
Calcium Carbonate Sujata Nutri Pharma limestone
This, in turn, varies according to what you're baking. But the simplest way to think of it is that the leavening agent produces the gas, and the gas causes the dough or batter to rise. There are three main types of leavening agents: biological, chemical, and steam.

Calcium Carbonate 96.8 APC Pure
Calcium carbonate is a type of naturally occurring calcium salt that is often used as a food additive, an antacid, a phosphate binder, or a dietary supplement. As a pharmaceutical product, it can.

Calcium Carbonate
Below are some common leavening agents: Ammonia (um) (Bi) Carbonate (hartshorn), Hydrogen Carbonate, Phosphate, Powered Baking, Dibasic, Monobasic. Beer (Beer in itself is NOT a leavening agent. Some processes can extract the yeast making the final product 'non-yeast'. Check the label to make sure.) Baking Soda: a crystalline alkaline salt.
Calcium Carbonate Molecule. it is an Ionic Compound, the Carbonic Salt
Leavening Agents. Leaven is any agent that produces fermentation and causes dough to rise, by causing the formation of carbon dioxide gas to bubble into and spread throughout the dough. This is accomplished either chemically (as with baking soda) or biologically (as with yeast).

Calcium Carbonate Powder (E170) The Melbourne Food Depot, Melbourne
As to fortification, the use of the calcium-based leavening components provides the benefit of extra calcium in the diet. CAPP when used in place of SAPP provides about a 25% decrease in sodium while at the same time providing a source of calcium, CAPP is about 19% calcium, which in many applications allows a claim of "good" or "excellent.

To Know Ground Calcium Carbonate from Six Aspects DASWELL
Process of Leavening •Leavening agents: -Also called leaveners. -Allow baked goods to rise. •Leavened baked goods: are lighter in. •Sources of calcium: calcium salts, such as calcium carbonate and calcium phosphate. Factors Affecting Yeast Fermentation 8. Amount of yeast. -More yeast means faster fermentation.
Lab test cement Black and White Stock Photos & Images Alamy
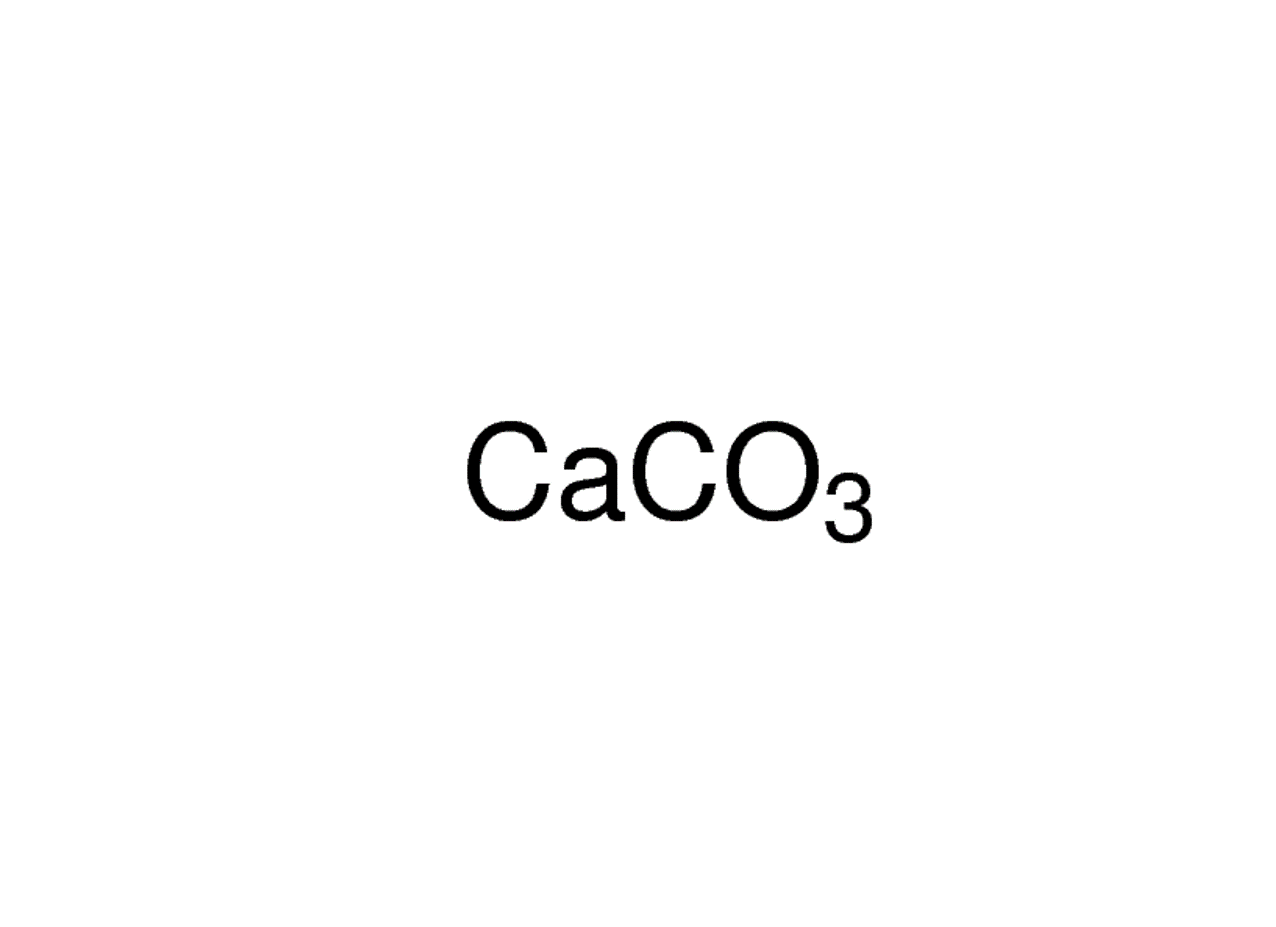
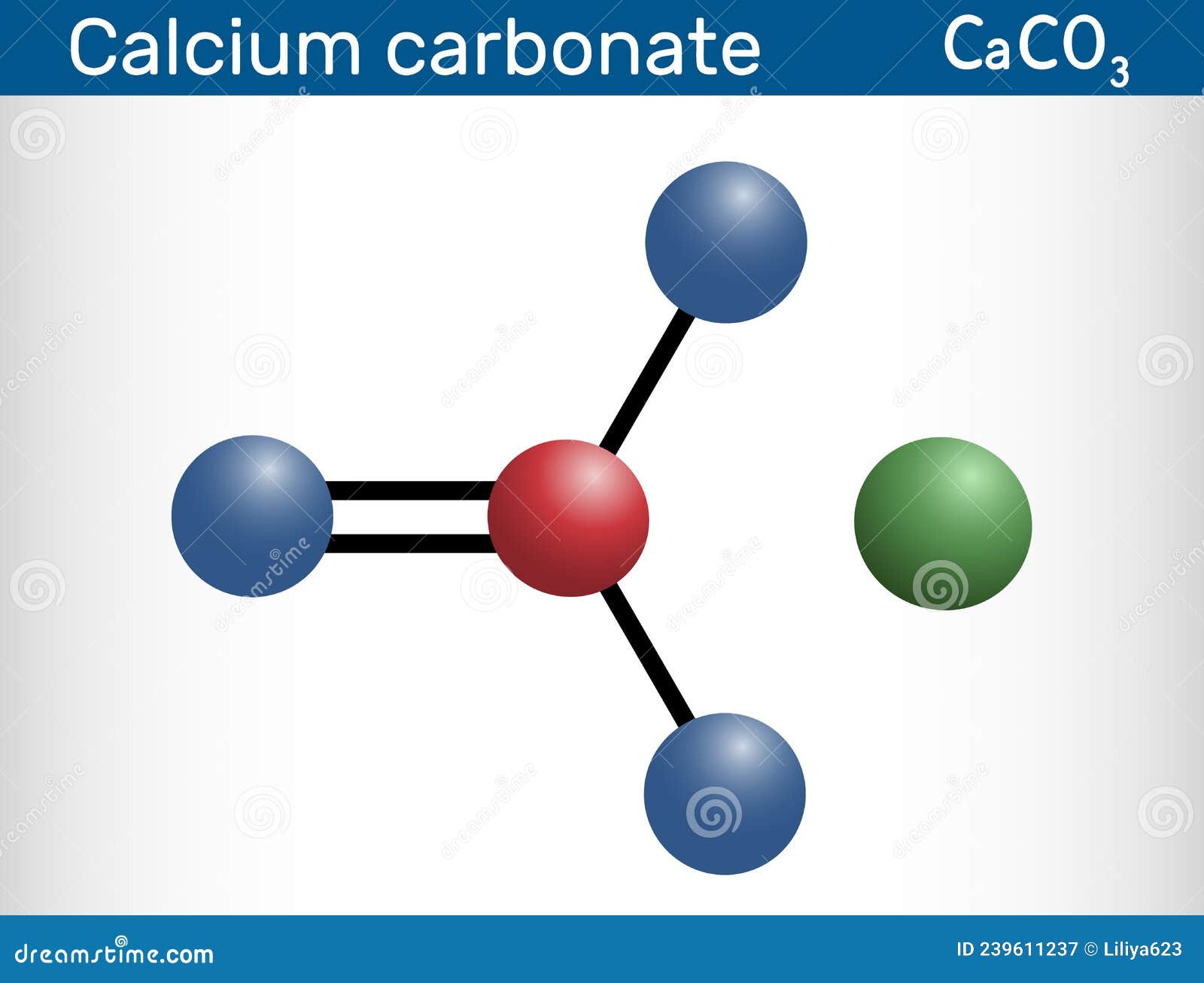
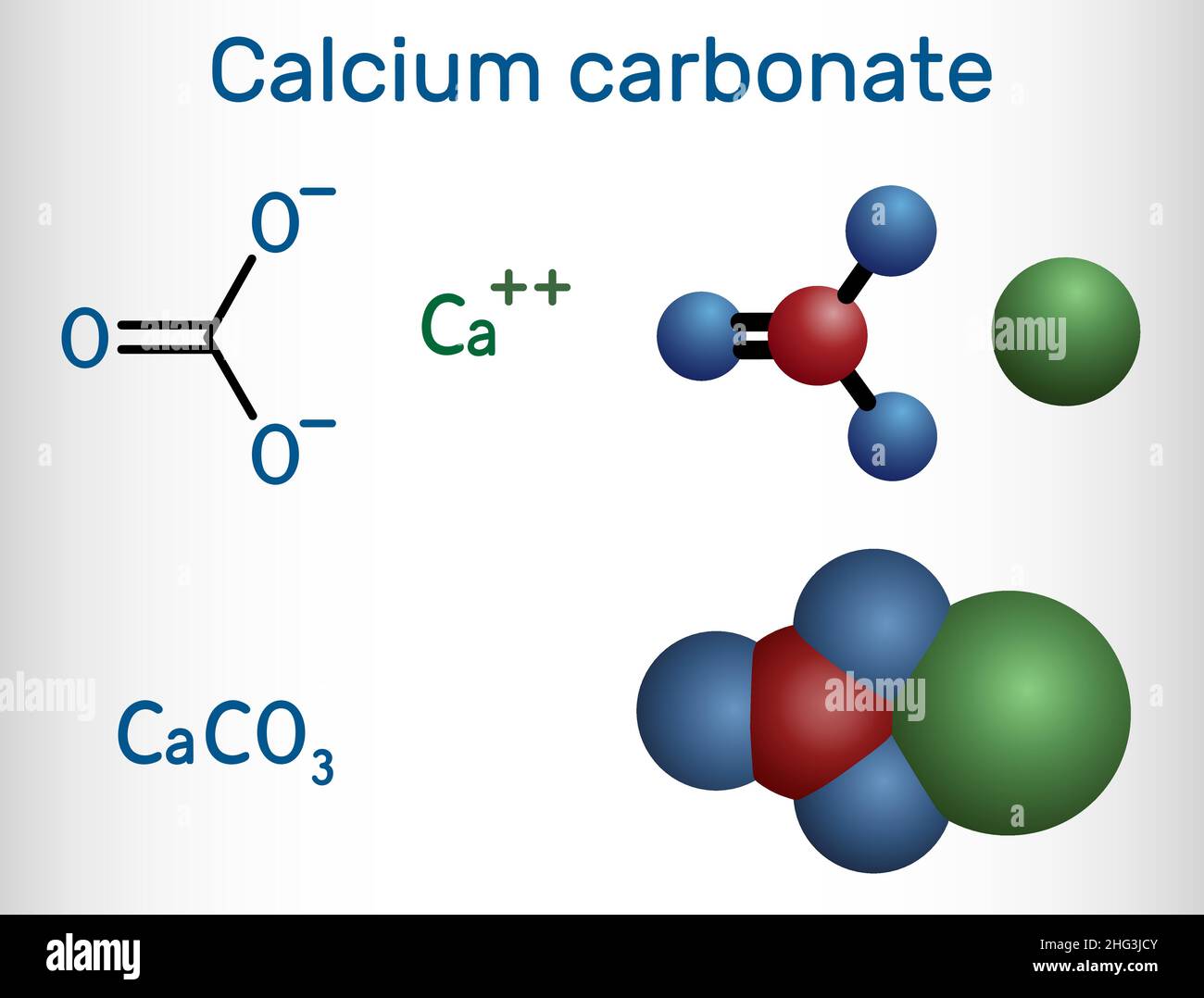
calcium carbonate (CaCO3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is the major constituent of limestone, marble, chalk, eggshells, bivalve shells, and corals. Calcium carbonate is either a white powder or a colorless crystal. When heated, it produces carbon dioxide and calcium oxide (also.

OSTEOBLOC Applied Pharmaceutical Distribution Inc.
The calcium and aluminum cations in phosphate-based leavening acids provide more resiliency to cake products than the sodium cation in other phosphate-based leavening agents. Therefore, if resiliency is a property desired in a cake product, the formulator may choose a leavening system containing calcium and/or aluminum ions.
Calcium carbonate molecule. It is an ionic compound, the carbonic salt
leavening agent, substance causing expansion of doughs and batters by the release of gases within such mixtures, producing baked products with porous structure. Such agents include air, steam, yeast, baking powder, and baking soda. Air and steam. Leavening of baked foods with air is achieved by vigorous mixing that incorporates air bubbles, producing foam.

slsi.lk how long for sulfatrim to work What is calcium carbonate
Crystal structure of calcite. Calcium carbonate is a chemical compound with the chemical formula Ca CO 3.It is a common substance found in rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls.Materials containing much calcium carbonate or resembling it are described as calcareous.
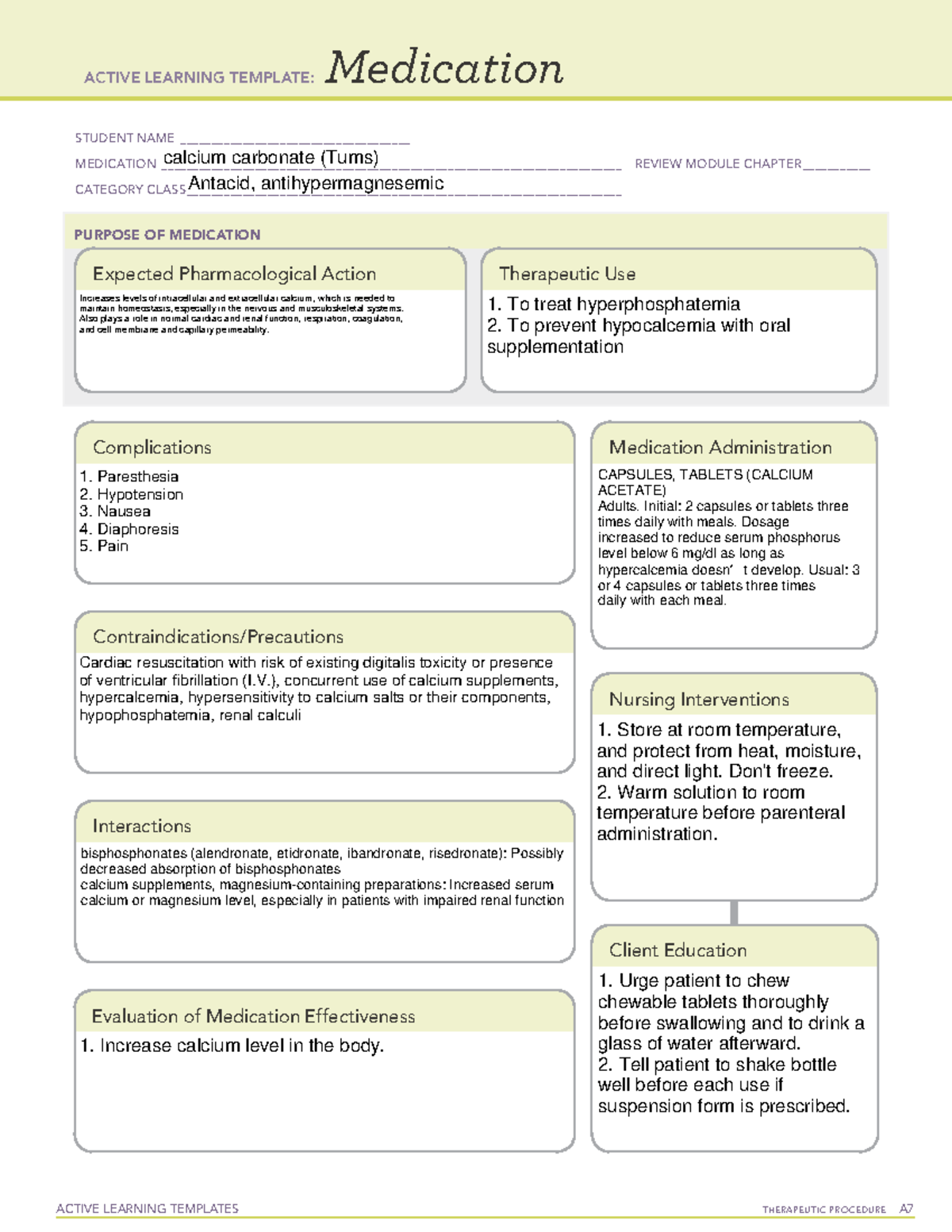
Calcium carbonate (Tums) ACTIVE LEARNING TEMPLATES THERAPEUTIC
Calcium carbonate is an inorganic salt primarily used to manage and treat low calcium conditions, GERD, CKD, and other indicated conditions. Calcium carbonate is classified as a calcium supplement, antacid, and phosphate binder. This activity outlines the significant indications, actions, and contraindications for calcium carbonate as a valuable agent in treating osteoporosis, hypothyroidism.