CH2Cl2 Lewis Structure Lewis Dot Structure for CH2Cl2 Dichloro

Is dichloromethane CH2Cl2 polar or nonpolar? Explain YouTube
Dichloromethane (DCM), also described as methylene chloride, is an organic molecule. This is generally used as a solvent in most organic reactions due to its polarity. Students generally ask question "Is CH2Cl2 polar or nonpolar?" DCM has the chemical formula CH2Cl2. It contains two hydrogen and two chlorine atoms in a tetrahedral structure.

Cloruro de diclorometano deuterado cloroformo deuterado, achtung
It is polar because of the presence of two chloro groups but is not miscible with water; however, it does show miscibility with various organic solvents such as chloroform, carbon tetrachloride, hexane, benzene, ethyl acetate, and alcohols.

MakeTheBrainHappy Is CH2Cl2 Polar or Nonpolar?
When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive and negative ends line up with the field. The magnitude of the turning force is given by the formula. µ = q × d. where q is the amount of charge and d is the distance between the two charges. µ is the turning moment.

Solved Question 9 (1 Point) What Are The Polarities Of CH...
CH2Cl2 is a POLAR molecule because the C-Cl bonds present in the molecule are polar, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire CH2Cl2 molecule polar.

Is CH2Cl2 Polar or Nonpolar? (Dichloromethane) YouTube
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.
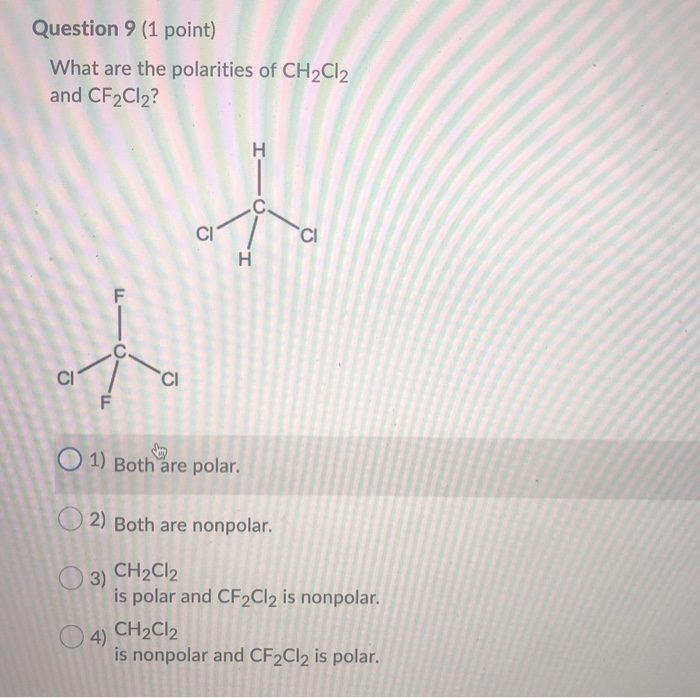
Solved Question 9 (1 Point) What Are The Polarities Of CH...
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.
Ch2cl2 3d Structure
To determine if CH 2 Cl 2 (dichloromethane) is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. Carbon is the central atom: There are 4 + 2 + 2×7 = 20 electrons, and 8 have been used to make four bonds.

CH2Cl2 Lewis Structure Lewis Dot Structure for CH2Cl2 Dichloro
Is ch2cl2 polar or is it non-nonpolar and whether it is ionic or covalent all these facts and characteristics have been discussed in detail in this article. We know ch2cl2 is a tetrahedral molecule and not all tetrahedral molecules are polar. But ch2cl2 is a polar molecule and almost all the tetrahedral molecules have a bond angle of 109.5 degrees.

Polar and Nonpolar Molecules
Also, polar solvents are better at dissolving polar substances, and nonpolar solvents are better at dissolving nonpolar substances. Figure 7.28 (a) Molecules are always randomly distributed in the liquid state in the absence of an electric field. (b) When an electric field is applied, polar molecules like HF will align to the dipoles with the.

Is ch2cl2 polar Why, How, When And Detailed Facts
I assumed, since the reason for CHX2ClX2 C H X 2 C l X 2 being polar is apparently due to the 109.5° angles from the tetrahedral shape, that this means that the surrounding atoms in all tetrahedral-shaped compounds are not placed directly opposite of each other and that is why the dipoles can't cancel each other out. Am I understanding this wrong?
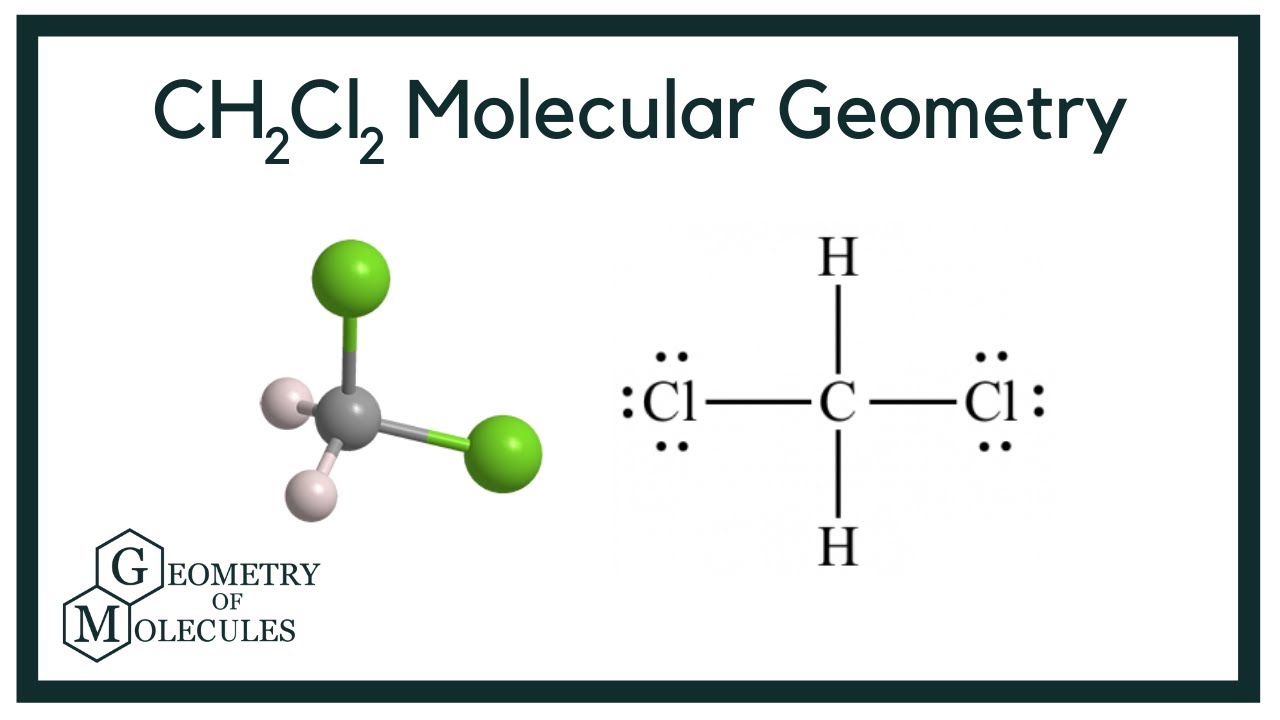
CH2Cl2 Molecular Geometry, Bond Angles & Electron Geometry
CH3Cl Polar or Nonpolar. To determine if CH 3 Cl is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. CH 3 Cl has 4 + 3 + 7 = 14 valence electrons. Carbon goes in the middle and is bonded to three hydrogen.

Best Explanation CH2Cl2 polar or nonpolar [N01] Science Education
Molecular Polarity. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms.

CH2Cl2 Lewis structure, Molecular geometry, Hybridization, Bond angle
Is CH2Cl2 Polar or Non-Polar? (Dichloromethane) Dichloromethane is a polar solvent. This means that it has a dipole moment, which is a measure of how strongly its molecules are attracted to one another.
Polar and Nonpolar Covalent Bonds Characteristics & Differences
Polar Or Nonpolar Dichloromethane (Ch2cl2) - Bond Angle, Molecular Geometry, And Hybridization. Dichloromethane (CH2Cl2) is a chlorinated chemical extensively employed as a solvent. It is among the least harmful chlorohydrocarbons, as well as miscible in a majority of organic solvents.
Is N2 Polar Or Nonpolar?
Is CH2Cl2 Polar or Nonpolar? (Dichloromethane) Wayne Breslyn 726K subscribers Join Subscribe Subscribed 373 Share 50K views 3 years ago Learn to determine if CH2Cl2 (Dichloromethane) is polar.
13+ Lewis Structure For Ch2Cl2 Robhosking Diagram
Exercise 2.12: Vitamins can be classified as water-soluble or fat-soluble (consider fat to be a very non-polar, hydrophobic 'solvent'. Decide on a classification for each of the vitamins shown below. Exercise 2.13: Both aniline and phenol are insoluble in pure water. Predict the solubility of these two compounds in 10% aqueous hydrochloric acid.